Preparation and characterization of α-Chitin nanofibers from crab shells of Portunus pelagicus (blue swimmer crab)
Keywords:
Chemistry, polymer, science, technology, sri lankaAbstract
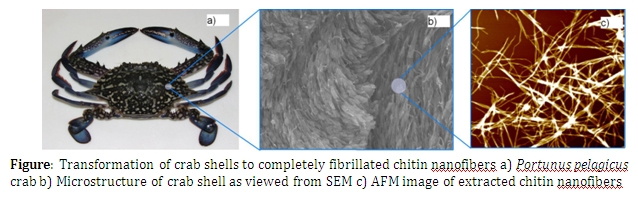
Chitin, the second most abundant biopolymer in the world after cellulose is found in a larger number of invertebrate species such as crustaceans and insects and in cell walls of fungi species. However chitin is primarily extracted from exoskeletons of crustaceans such as prawns, shrimps and crabs. Chemically, chitin is a linear amino-polysaccharide known to be made of 2-acetamido-2-deoxy-β-D- glucopyranose through a β(1→ 4) linked structure. The main functionality of chitin in most biological systems is to provide structural rigidity and strength. In crab shells however, a very complex formation of chitin nanofibers, called Bouligand structure, can be observed. This structure follows a twisted plywood like pattern providing additional stiffness and hardness to the shell.
The purpose of this work is to extract chitin nanofibers from crab shells of Portunus pelagicus in its elementary scale. During the course of study the crab shell structure was examined using Scanning Electron Microscopy (SEM) imaging. This has confirmed that chitin exists in nanofiber form having diameters in the size range of 3-5 nm in the structure. These nanofibers arrange themselves into larger bundles having 60 – 70 nm in diameter.
Unlike in conventional chitin extraction procedures where chitin nanofibers collapse to form a matrix where separation of nanofibers to individual level is impossible, we have used an ultrasound assisted procedure to achieve complete fibrillation of chitin nanofibers. Surface morphology of prepared nanofibers was evaluated using Atomic Force Microscopy (AFM) and SEM. Results suggested that almost all the nanofibers prepared, were completely fibrillated and well dispersed. Crystallinity of the nanofibers was evaluated using X-Ray Diffraction (XRD) and found to follow the α-chitin form and the crystalline nature has not been disturbed during the process. The degree of deacatylation was calculated using Fourier Transform Infar-Red spectroscopy (FTIR) and the thermal character of prepared nanofibers was evaluated using Thermo Gravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC). The analysis confirmed that prepared nanofibers had good purity with degree of deacetylation less than 15%.
Key words: Chitin / Nanofibers / Extraction / Fibrillation
